Sequestration Suspension Extended: Impact on Drug Reimbursement
Pharmacy Revenue Cycle Podcast
Release Date: 01/04/2022
 The Heavy Hitters for July Quarterly Updates
The Heavy Hitters for July Quarterly Updates
Pharmacy Revenue Cycle Podcast
July 2023 is here and time to validate another round of quarterly updates from CMS. The JZ modifier, in addition to the JW modifier, is now required to effectively bill for drug waste (JW) and to attest when no drug was discarded (JZ) for all separately payable that are single-dose or single-use containers. Additionally, we have updated the Visante Quarterly Update Tool and the C9399 Tool to help organizations validate that their system is up to date with the recent changes.
info_outline Beat Inflation with a Part B Rebate
Beat Inflation with a Part B Rebate
Pharmacy Revenue Cycle Podcast
As a part of the Inflation Reduction Act of 2022, CMS is requiring manufacture rebates for certain Medicare Part B drugs in which the cost has exceeded inflation. Beneficiaries out of pocket costs will be reduced to 20% of the inflation-adjusted payment described in the Act.
info_outline Don’t be “SAD”... An Alternative Way to Handle Self-Administered Drugs
Don’t be “SAD”... An Alternative Way to Handle Self-Administered Drugs
Pharmacy Revenue Cycle Podcast
Self-administered drugs (SAD) have been a long-standing controversy when administered in a hospital outpatient setting from the perspectives of a patient, frontline healthcare workers, and billing. “Why does my Tylenol cost $10 per tablet, but the 1,000-count bottle I have at home was purchased for $3?” This question is often difficult to answer and may lead to unintended operational consequences.
info_outline Botulinum toxin PA – Are you exempt?
Botulinum toxin PA – Are you exempt?
Pharmacy Revenue Cycle Podcast
The new year brings a new focus on resolutions including prior authorization processes. In July 2020, Medicare implemented a prior authorization process for select services including botulinum toxin. Its time to revisit workflow processes and, if not exempt, confirm with respective teams that a prior authorization is obtained prior to providing the service.
info_outline Designer HCPCS Codes are in the Mainstream Spotlight
Designer HCPCS Codes are in the Mainstream Spotlight
Pharmacy Revenue Cycle Podcast
The Pharmacy Revenue Cycle is starting out with a new fashion design for 2023 as there are 36 new brand-specific HCPCS codes. CMS has been reviewing its approach for assigning for drugs that have been approved under the Food, Drug and Cosmetic Act 505(b)(2) New Drug Application (NDA) or the Biologics License Application (BLA) after October 2003. These drugs are not rated therapeutically equivalent to the reference drug listed in the FDA’s Orange Book and therefore are considered single-source products according to section 1847A(c)(6) of the Social Security Act. Each single source product...
info_outline Hospital Outpatient Prospective Payment System (OPPS) Final Rule- CY2023
Hospital Outpatient Prospective Payment System (OPPS) Final Rule- CY2023
Pharmacy Revenue Cycle Podcast
The Centers for Medicare & Medicaid Services (CMS) provided the OPPS Final Rule for CY2023 in the Federal Register on Provisions in this rule will be effective for dates of service on or after January 1, 2023. Significant changes for drug reimbursement and coding occur in three areas: 340B-acquired drugs, non-opioid pain management reimbursement in Ambulatory Surgery Centers (ASC) and Hospital Outpatient Departments (HOPD), and new requirements for reporting waste in HOPD. 340B-acquired Drugs In light of the Supreme Court decision in American Hospital Association v. Becerra, 142 S. Ct....
info_outline Drug Waste is Packed with a Punch and a Refund
Drug Waste is Packed with a Punch and a Refund
Pharmacy Revenue Cycle Podcast
Dive into the CY23 CMS Physician Fee Schedule rule as it relates to the new requirements for discarded drugs or drug waste. A JW and JZ modifier are required for all Part B separately payable single-dose or single-use packages. Additionally, manufacturers are required to pay a refund for discarded drugs that exceed 10% of the total charges.
info_outline Payment Increases for Biosimilars
Payment Increases for Biosimilars
Pharmacy Revenue Cycle Podcast
Payment Increases for Biosimilars On April 16th, 2022, the Inflation Reduction Act of 2022 was signed into law. Section 11403 requires a temporary increase in add-on payment for qualifying biosimilars from 6% to 8% for 5 years. This change was implemented on October 1, 2022, and CMS uploaded pricing files that already include the temporary price increase. Applicable 5-year period This increase began on October 1, 2022, for products for which payment was made by September 30, 2022. For other biosimilar products in which payment was made between October 1, 2022 - December 31, 2027, the 5-year...
info_outline Car T-cell Therapy: Coverage and Billing-Outpatient (Updated – October 1, 2022)
Car T-cell Therapy: Coverage and Billing-Outpatient (Updated – October 1, 2022)
Pharmacy Revenue Cycle Podcast
Chimeric Antigen Receptor (CAR) T-cell therapy is an example of a rapidly emerging immunotherapy approach called adoptive cell transfer (ACT) where patients’ own immune cells are collected and used to treat their cancer. This newsletter details coverage and billing instructions when the products are used on an outpatient basis and has been updated to reflect HCPCS codes current as of October 1, 2022. The Center for Biologics Evaluation and Research (CBER) of the Food and Drug Administration (FDA) regulates cellular therapy products, human gene therapy products, and certain devices related to...
info_outline Vacating the 340B Payment Reduction Policy
Vacating the 340B Payment Reduction Policy
Pharmacy Revenue Cycle Podcast
On September 28, 2022, the US District Court issued a that states the Department of Health and Human Services (HHS) is required to vacate the prospective portion of the 340B reimbursement rate outlined in the 2022 Outpatient Prospective Payment System (OPPS) Rule. In other words, payment rates must revert to the default of ASP + 6% rather than the reduced rate for select drugs of ASP - 22.5%. The decision was determined to not cause substantial disruption; thereby, requiring HHS to begin immediately. This was in response to American Hospital Association v. Becerra, 142 S. Ct. 1896 (2022), in...
info_outlinePharmacy departments may periodically be asked to compare drug payment with cost, particularly when expensive drugs are utilized on outpatients. It is also important to calculate anticipated revenue when preparing annual budgets.
The ASP (Average Sales Price) file is published by CMS each quarter and can be used to verify payment for Medicare outpatients or commercial contracts which pay based upon Medicare payment. It sounds straightforward, but there is a catch: “sequestration”.
Sequestration is the automatic reduction of federal spending originally established by Congress in the Balanced Budget and Emergency Deficit Control Act of 1985 (BBEDCA, also known as the Gramm-Rudman-Hollings Act). This applies to most areas of the federal budget but was first “triggered” for Medicare in FY2013 resulting in a 2% reduction in Medicare payments to providers over stated payment rates. This 2% reduction has continued each fiscal year until the pandemic when the sequestration was “suspended” meaning there is no current payment reduction and provided receive the full payment.
The Protecting Medicare and American Farmers from Sequester Cuts Act was signed into law on December 10, 2021 and extends the suspension through March 31, 2022. However, the sequestration will be gradually applied once the extension expires. From April 1, 2022 through June 30, 2022, the payment adjustment will be 1% and effective July 1, 2022, the payment adjustment shall return to the 2% for all Medicare Fee-for-service claims. Any changes to this reinstatement would require further Congressional action.
Because Medicare beneficiary co-payments are not subject to the payment reduction, they continue to pay the same co-payment amounts regardless of sequestration. Here’s a formula to use when the “sequestration” suspension begins to expire on April 1, 2022:
((Payment rate-copayment)*0.99)+ copayment
Let’s take a drug example from January 2022. Using Addendum B, we see the following:
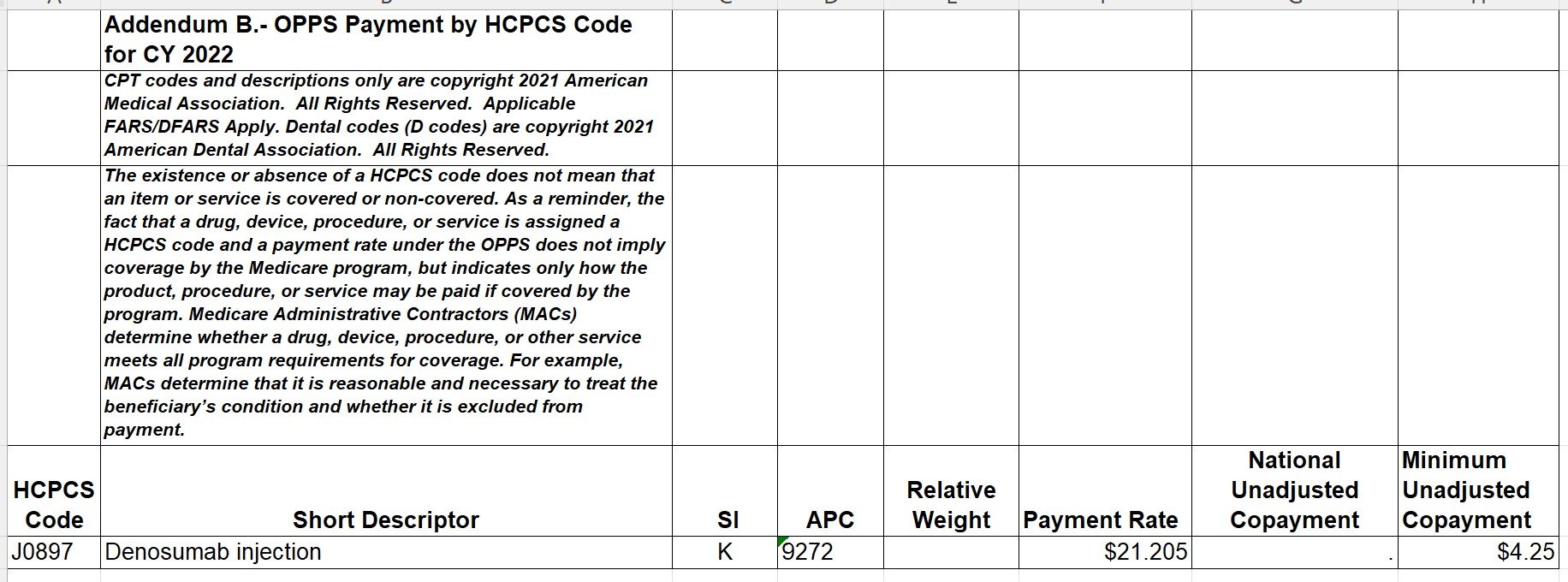
Since Addendum B doesn’t have the dose description for this HCPCS code, we can find that information on the ASP file for the quarter:
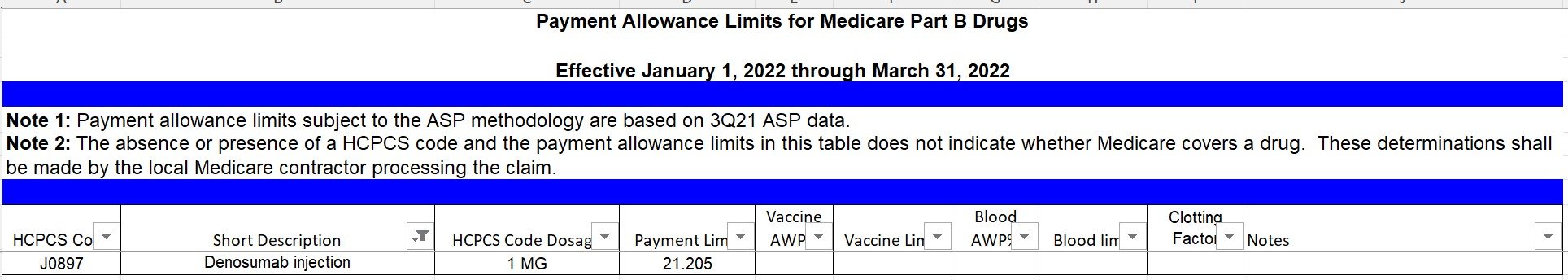
Denosumab, J0897 has a payment limit of $21.205 per 1 mg effective January 1, 2022 from the ASP file.
Calculating the new payment rate when subject to a 1% sequestration results in the following:
(($21.205-$4.25)*0.99) + $4.25 = $21.035
For a 60 mg dose, the payment difference is $10.173 (($21.205-$21.035)*60))
Note: The rates reflected in Addendum B are “unadjusted copayments” and these co-pay amounts may be different for different providers based upon geographic adjustments.
SHOUT- OUTS
-
Pharmacy and Finance should take into consideration if there is a payment “sequestration” triggered when projecting budgets and business plans especially for expensive drugs used on outpatients.
-
Pharmacy and Finance should be aware of congressional actions which may impact drug payments.
Our goal is simple; we’re taking complex information and making it practical.
Until our next edition, this is Maxie Friemel and Agatha Nolen providing you with tips for increasing your Pharmacy Revenue.
